
Failure Mode Effects Analysis (FMEA)
 Failure
Mode Effects Analysis (FMEA) or to give it its correct title Failure Mode
Effects & Criticality Analysis (FMECA) is a logical technique used to identify
and eliminate possible causes of failure. The technique requires a sequential,
disciplined approach, to assess systems, products or processes in order to
establish the modes of failure and the effects of failure on the system, product
or process. This is to ensure that all possible failure modes have been fully
identified and ranked in order of their importance. The FMECA discipline
requires the documentation (see worksheet opposite) of any evaluation with
regard to the failure mode, effect and criticality. The analysis work can be
applied at any stage; design, manufacture, test, installation or use, but is
best performed at the early (development or design) stage. In a simple system
the study may be performed on the total system or product but with more complex
systems it may be necessary to break the product down into various sub-systems
or sub-assemblies.
Failure
Mode Effects Analysis (FMEA) or to give it its correct title Failure Mode
Effects & Criticality Analysis (FMECA) is a logical technique used to identify
and eliminate possible causes of failure. The technique requires a sequential,
disciplined approach, to assess systems, products or processes in order to
establish the modes of failure and the effects of failure on the system, product
or process. This is to ensure that all possible failure modes have been fully
identified and ranked in order of their importance. The FMECA discipline
requires the documentation (see worksheet opposite) of any evaluation with
regard to the failure mode, effect and criticality. The analysis work can be
applied at any stage; design, manufacture, test, installation or use, but is
best performed at the early (development or design) stage. In a simple system
the study may be performed on the total system or product but with more complex
systems it may be necessary to break the product down into various sub-systems
or sub-assemblies.
The technique is often seen as part of an organisation's; Lean, Value Stream Analysis, Root Cause Analysis programme.
Reasons for FMECA
| With ever increasing demands to ensure that QUALITY is achieved RIGHT
FIRST TIME then still greater pressures are placed on the engineer or
process developer. This is to ensure that the process or design performs
consistently, reliably and safely throughout the life of the product or process,
thus providing a quality product or service that completely meets the demands of
the customer. Designers and developers are only human, they can make mistakes
and have off days just like everyone else. FMECA ensures that any inadequacies
in the product or service are quickly identified, preventing the possibility
of releasing sub-standard products or providing a sub-standard service.
Product testing will of course help identify any design deficiencies, there
are however, possible limitations with this approach:
Other reasons may include, meeting the requirements of:
|
|
So FMECA provides the potential for:
| Reducing the likelihood of Customer Complaints | |
| Reducing the likelihood of campaign changes | |
| Reducing maintenance and warranty costs | |
| Reducing the possibility of safety failures | |
| Reducing the possibility of extended life or reliability failures | |
| Reducing the likelihood of product liability claims |
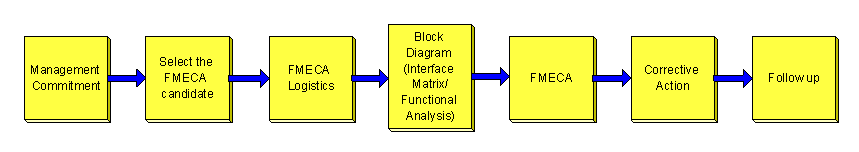
Typical FMECA Process
![]()
For further information
| Training courses | |
| Software | |
| FMEA Support | |
| FMEA Free Software |
If you would like to know more about FMEA please contact Sales at:
General Information: sales@qmt.co.uk
![]()
Links